Beyond the device: A day in the life of a Senzime Clinical Product Specialist
Nov 26, 2025
Blog by Daniel Puhl, BSN, RN, Clinical Product Specialist at Senzime
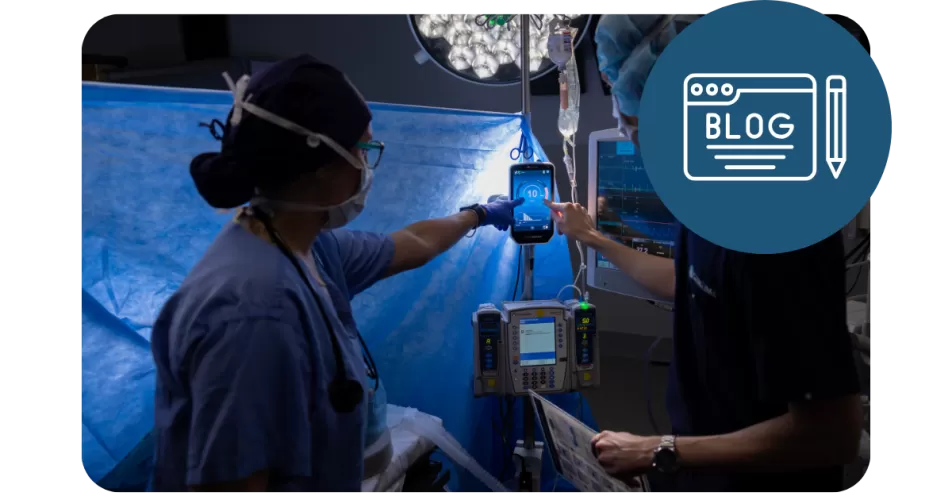
At Senzime, technology is only half the story — the other half is people. Our Clinical Product Specialists (CPSs) bridge innovation and real-world care, ensuring that every TetraGraph® monitor helps to support safer, more accurate neuromuscular block management.
Every Senzime Clinical Product Specialist brings a mix of clinical and technical expertise. All are ICU-trained nurses who can translate device data into meaningful, bedside decisions. They’re equally comfortable explaining EMG-based algorithms as they are supporting an anesthesia clinician through a case. The role demands agility — every OR and ICU is different — and the ability to communicate clearly with a diverse range of clinicians, from anesthesiologists and CRNAs to anesthesia techs and nurses.
The work is highly dynamic. Early in an implementation, Clinical Product Specialists are often on-site, guiding teams through setup, training, and adoption. As accounts mature, the role evolves into a more consultative position— analyzing usage patterns, addressing challenges, and reinforcing best practices.
The goal is always the same: helping every facility achieve confident and consistent use of quantitative train-of-four (TOF) monitoring.
Senzime’s clinical support extends far beyond device repair. Our Clinical Product Specialists provide end-to-end guidance from demos through long-term adoption. Because our team is clinician-led, we speak the language of anesthesia and proactively monitor customer feedback and sensor utilization to identify gaps and offer solutions to fit your workflow. We see ourselves as your partners in patient safety.
Change in the OR is never simple. Clinicians may be skeptical of new technology or need time to adapt workflows. Clinical Product Specialists address those challenges directly — teaching, troubleshooting, and building trust. Whether it’s refining sensor placement, clarifying physiology and “buttonology,” or simplifying data interpretation, our goal is to highlight how our TetraGraph technology is intuitive and reliable.
At Senzime, clinical support is part of the product. Our lifecycle approach means we never “hand you off” after installation. Insights from the field directly shape product updates, education modules, and the ongoing training efforts. This continuous feedback loop enables us to identify barriers early and optimize outcomes more efficiently.
Hospitals and anesthesia teams see the results:
- Improved patient safety with reduced residual neuromuscular block
- Higher ROI through consistent device use
- Less strain on hospital resources — from IT to biomed to anesthesia techs
- Faster adoption and greater clinician confidence
At Senzime, a Clinical Product Specialist's day might include supporting a clinical trial, managing a new installation, or returning to strengthen adoption at an existing site. At the core of every visit is partnership.
- As advocates, we use evidence-based practice to promote safer anesthesia recovery.
- As educators, we teach in real clinical environments — from ORs to ICUs — ensuring every clinician can integrate the TetraGraph into their workflow with ease.
- As troubleshooters, we fine-tune details to make every interaction effortless.
- As liaisons, we connect departments, including anesthesia, biomed, and IT, and bring hospital feedback back to Senzime to drive continuous improvement.
In complex perioperative environments, trust matters. When clinicians see that we understand their world and share their goals, they know our advocacy and education come from an authentic desire to improve outcomes.
For Senzime’s Clinical Product Specialists, every visit, every case, and every conversation revolves around one mission: delivering safer, smarter care — one patient at a time.
Learn how our Clinical Product Specialists can support your team from installation to everyday excellence.
Explore our support resources or connect with us to start the conversation.
Daniel Puhl, BSN, RN is a Clinical Product Specialist at Senzime

