From Tradition to Evidence: The Case for ADM in Neuromuscular Monitoring
Sept 9, 2025
Blog by Jen Sanders, Director of Clinical & Medical Affairs at Senzime
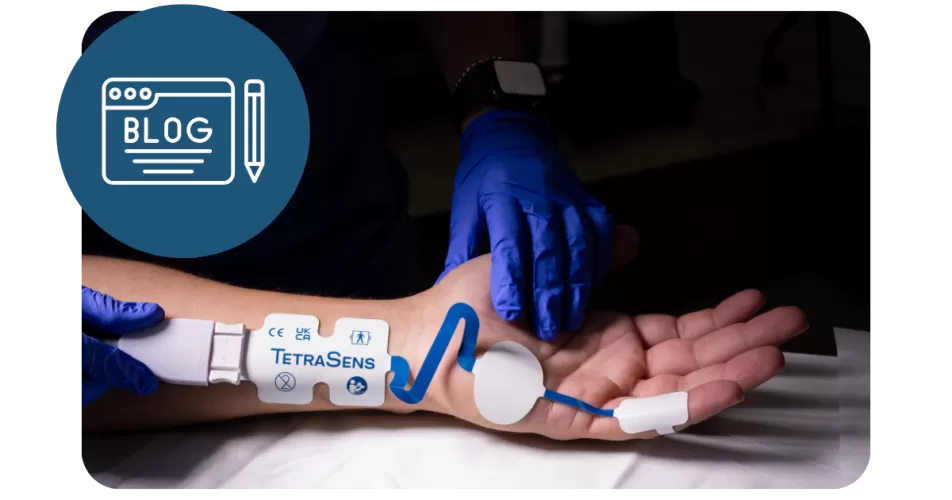
Most anesthesia guidelines point us to the adductor pollicis (AP) muscle when monitoring neuromuscular block. But here’s the question: why does the adductor digiti minimi (ADM)—a site that evidence shows is just as reliable—rarely get a mention?
The answer lies not just in clinical evidence, but in history.
For decades, the “gold standard” in neuromuscular monitoring was mechanomyography (MMG).
- MMG measures the force of muscle contraction in response to nerve stimulation.
- To obtain reliable results, MMG required a muscle/nerve pairing that minimized interference from direct muscle stimulation (contractions caused by stimulating the muscle fibers directly, not via a nerve).
The ulnar nerve and the adductor pollicis (AP) muscle proved to be the ideal combination. Here’s why:
- The AP is the only hand muscle on the radial (thumb) side innervated by the ulnar nerve.
- This unique anatomy allowed a clean, uncontaminated signal with MMG.
Because of this, AP became deeply ingrained in both research protocols and clinical guidelines.
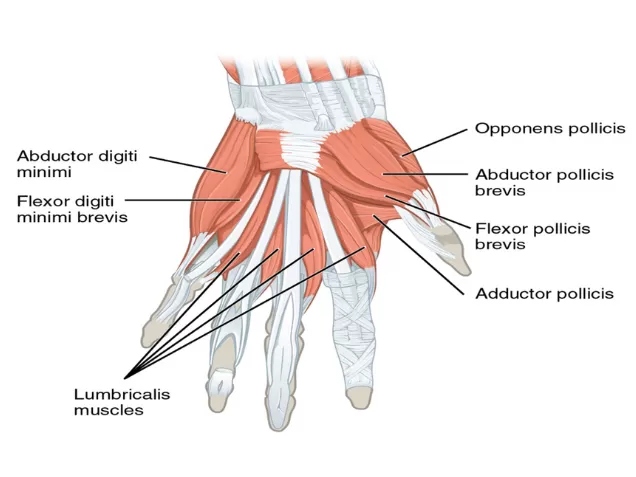
While AP has long been the “default” monitoring site, there are important anatomical and practical challenges that can affect accuracy.
Image credit: CFCF, “Thenar and hypothenar eminences,” CC BY-SA 4.0, via Wikimedia Commons.
- The AP is actually a small muscle located deep to the abductor pollicis brevis, flexor pollicis brevis, and opponent pollicis muscles. As a result, electrode placement is rarely optimal. Thumb movement may be elicited but often reflects a combination of contractions from surrounding muscles rather than a pure AP signal.
Moreover, the AP itself consists of two separate muscle heads (oblique and transverse) that contract in different directions—meaning stimulation may preferentially activate one head or the other, reducing consistency.
- During ulnar nerve stimulation, the entire thumb contracts and adducts toward the palm, shifting the AP’s position relative to the recording electrode with each contraction.
This movement can alter the amplitude of the recorded signal, further affecting reliability and repeatability.
- In the operating room, practical factors come into play. Patient hands are often tucked under drapes, and perspiration is common in the palms (especially at the thenar and hypothenar eminences).
With each contraction, the thenar eminence moves substantially making AP electrodes more likely to loosen or dislodge. In contrast, the hypothenar eminence (where ADM electrodes are placed) is far more stable, reducing signal variability or electrode displacement during surgery.
When acceleromyography (AMG) and kinemyography (KMG) entered the field, they inherited the same principle:
- Both rely on muscle movement as their measurement signal.
- To remain accurate, they also needed to minimize contamination from direct muscle stimulation.
Once again, the ulnar nerve/AP muscle combination was the most reliable option. This further reinforced AP’s position as the “go-to” site.
With electromyography (EMG), the physiology and the measurement methods are different:
- EMG does not measure muscle contraction or movement—it measures electrical conduction and receptor function in response to nerve stimulation.
- Because the signal reflects nerve and receptor activity, it is not contaminated by direct muscle stimulation.
This means that alternative muscles, such as ADM, can be monitored just as effectively as AP.
And recent research backs this up:
- Phillips et al. (2012) found that with EMG, ADM was the most resistant to block and the most precise, with narrower limits of agreement at TOF 0.9 compared with AP.
- Iwasaki et al. (2022) showed that AP with AMG overestimated recovery, while ADM with EMG provided a more reliable assessment for reversal dosing.
- Nagy et al. (2023) reported no significant differences in onset or recovery between AP and ADM when both were monitored with EMG, confirming that either site is clinically valid.
So why do many guidelines still highlight AP and not ADM? It’s mainly due to history and tradition. The recommendations were established in the MMG era and carried forward into AMG/KMG practice.
As EMG monitoring becomes more widespread and as new evidence accumulates, it’s likely that future guidelines will begin to acknowledge ADM alongside AP. For now, understanding the why behind the recommendations helps clinicians apply the most effective monitoring strategies for their patients.
AP appears in guidelines not because ADM is inferior, but because historical technology required it.
With EMG, both AP and ADM are valid sites—opening the door to greater flexibility, precision, and patient safety in clinical practice. The limitation is not in which hand muscle to monitor, but in not using a quantitative monitor.
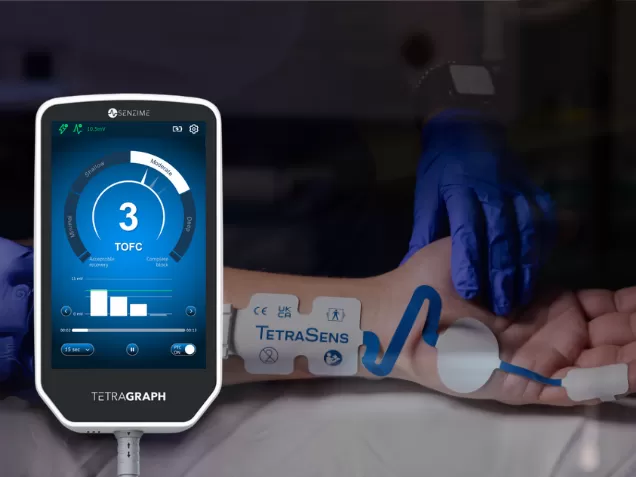
Whether you are a proponent of AP or ADM, the beauty of Senzime’s TetraGraph system is its unique ability to adapt to the clinician’s choice to benefit the patient.
- Flexible placement: TetraGraph EMG sensors can be applied to multiple monitoring sites—hand or foot—depending on patient needs and clinician preference.
- Consistent accuracy: Whether monitoring AP, ADM, or FHB (flexor hallucis brevis) muscles, the system delivers reliable, high-quality quantitative data.
- Clinical control: With TetraGraph, clinicians have the freedom to select the optimal site without compromising accuracy or safety – the clinician has control.
Some EMG-based monitoring systems highlight "automatic site selection" as a differentiator. While that may sound convenient, it removes clinical judgment from the process. With the next-generation TetraGraph®, the clinician (not the device) decides the optimal monitoring site. This preserves flexibility, respects clinical expertise, and ensures patient-specific precision monitoring.
Me and my colleagues are happy to guide you and answer any questions you might have.
Jen Sanders, Director of Clinical & Medical Affairs at Senzime

Learn about the latest clinical guidelines, get started with quantitative TOF monitoring, and access valuable insights to improve patient outcomes.
